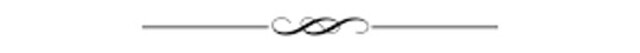Previously, researchers knew that depriving mice of sleep after the mice performed a task resulted in the mice forgetting aspects of that task. But researchers weren’t sure what function of the hippocampus—two seahorse-shaped structures located in the temporal lobe of the brain where many long-term memories are made—couldn’t do its job.
Now, researchers have found that interfering with sleep-associated oscillations—or the rhythmic firing of neurons—in one subsection of the hippocampus is likely the culprit. Their results appear in Nature Communications.
To test the role of oscillations in memory formation, the researchers recorded the baseline hippocampal activity of a group of mice. They placed mice into a new environment, let them explore, gave them a mild foot shock, then put them back into their home cages to rest and sleep normally.
“If you return the mouse to that same structure a day or even a couple months later, they will have this very stereotyped fear response, which is that they freeze,” says senior author Sara Aton, an assistant professor in the University of Michigan’s molecular, cellular, and developmental biology department. “But if you sleep-deprive an animal for a few hours after that context-shock pairing, the mouse won’t remember it the next day.”
The researchers found that in normally sleeping mice, sleep-associated oscillations in a subsection of the hippocampus called CA1 were more robust after learning. They then took a new group of mice, recorded their baseline hippocampal activity and had them complete the same task. The researchers also gave these mice a drug to inhibit a small population of inhibitory neurons in CA1 that express parvalbumin.
The researchers didn’t change the sleep behavior of the animal—they slept normally. But turning off the activity of parvalbumin-expressing neurons disrupted the rhythmic firing of surrounding CA1 neurons while those animals were asleep. Suppressing the parvalbumin-expressing cells appeared to completely wipe out the normal learning-associated increase in oscillations in that section of the mouse’s hippocampus.
“There’s an old theorem called Hebb’s Law, which is, ‘Fire together, wire together,'” Aton says. “If you can get two neurons to fire with great regularity in close proximity to each other, it’s very likely you’re going to affect the strength of connections between them.”
When the neurons were kept from firing together regularly and rhythmically, the mice forgot there was any fearful association with their task.
“The dominant oscillatory activity, which is so critical for learning, is controlled by a very small number of the total cell population in the hippocampus,” says Nicolette Ognjanovski, a graduate student and coauthor of the study. “This changes the narrative of what we understand about how networks work. The oscillations that parvalbumin cells control are linked to global network changes, or stability. Memories aren’t stored in single cells, but distributed through the network.”
The researchers also compared the stability of the neurons’ connections between the control group and the group whose sleep oscillations were disrupted. They found that not only were the connections stronger in the control group after their learning trial, but also that those neuronal connections were also stronger. These changes were blocked when sleep-associated hippocampal oscillations were experimentally disrupted.
“It seems like this population of neurons that is generating rhythms in the brain during sleep is providing some informational content for reinforcing memories,” Aton says. “The rhythm itself seems to be the most critical part, and possibly why you need to have sleep in order to form these memories.”
Next, the researchers plan to test whether restoring hippocampal oscillations (mimicking the effects of sleep in CA1) is sufficient for promoting normal memory formation when mice are sleep-deprived.
Source: University of Michigan
Related Books
at InnerSelf Market and Amazon